Our partner NATA-accredited laboratory, Sydney Laboratory Services, sheds some light on the useful analytical technique of gas chromatography and how it operates in compound identification.
What is Gas Chromatography?
Gas chromatography covers an extremely wide range of analytical techniques that laboratories use to identify compounds in samples. In short, gas chromatography is the art of separating compounds out for detection. By itself, gas chromatography is not a very useful tool. However, when paired with detectors, we can generate useful qualitative and quantitative results.
Gas chromatography is made up of three different aspects:
- Injection Port: Where the sample is introduced into the instrument
- Column/Oven: Where the sample is passed through to separate out compounds
- Detector: Where individual compounds create signals that are used to quantify results
Injection Port
This is the first part of the gas chromatography process. Samples will be initially treated with organic solvents to extract any soluble compounds that the laboratory wishes to identify. Each test will have its own specific solvents and extraction procedures, depending on what analysis type is requested.
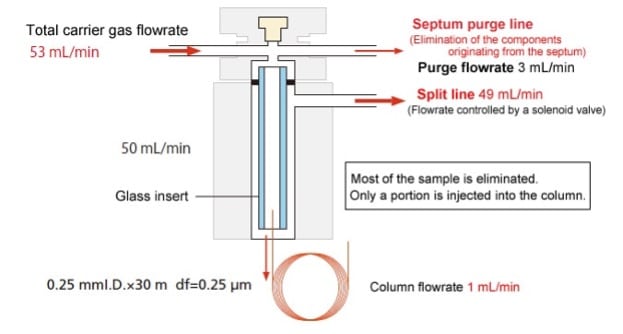
Once extracted, samples are placed in 2mL vials and transferred to the autosampler. The autosampler consists of a carousel (for sample storage) and an injector with an injection syringe. Generally, the lab will inject 1μL of sample into the injection port.
After being introduced into the injection port, the laboratory uses a split ratio to further dilute the sample, for every 20 parts of sample injected, only one part will pass through to the column.
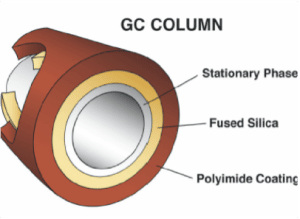
Column/Oven
After injecting, the sample will subsequently pass through to the gas chromatography Column, located within the gas chromatography Oven. The column, connected to the injection port, has a carrier gas running through it, the most common gas being Helium. However, Hydrogen, Nitrogen, and even air can be used. Each gas will have its own advantages and disadvantages, depending on the analysis types requested. Nevertheless, in practice, most laboratories will use Helium, owing to its safety, versatility, and cost.
Once the sample passes into the column, it is ‘carried’ by the carrier gas. A gas chromatography column will generally have a diameter of 0.100mm, a length of 10-30m and an internal coating 0.1μm thick. This internal coating is specially formulated for each type of compound group being analysed. Each compound will interact with the internal coating and the carrier gas, in differing amounts as they “flow” through the column. Some compounds will “stick” or interact with the insides of the column and take longer to pass through, whereas others will interact less and pass through faster. This is how the laboratory achieves separation. The time between compounds eluting from the column is called resolution, with the lab trying to always achieve the maximum resolution to help with compound identification, without taking too much time for a single sample to run.
Resolution can be affected by many different factors including:
- Column dimensions: length, internal diameter and the type of coating
- Oven temperature: higher temperatures allow compounds to “flow” faster
- Carrier gas: the type of gas and the velocity of the gas
Once eluted from the column, the compounds pass onto the detector.